Enrollee tasks and what these require
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
Here is an assembled list of what enrollees will carry out.
Some will decide to eschew participation because what follows seems extensive.
Others will understand that these required tasks represent a process that should clarify if a specific intervention with light is of any use to those suffering "long-term" COVID-19, or not.
Part of the work is carrying out the intervention with a specific light source.
The other part is gathering and transmitting the data to allow analysis of potential effectiveness or lack of benefit. Clearly important: no data, no results, no analysis, no meaningful answer.
-
- Participation requires committing to 31 days in sequence of interventions and measurements once begun. One cannot take a week off then come back to it. (If that already seems rather long, the last 10 days are "days off" or rest days. So 21 sequential days of intervention and study).
- "The intervention" is guided by a process of randomization of enrollees. Not all will be doing the same thing. Nevertheless each arm of the study (EA, 20 individuals) and (NEA, 20 individuals) will carry out identical components as follows:
-
-
- "Intervention group": 10 minutes of structured exposure to light passed over the head region. (10 individuals in each of the two arms).
- "Intervention + group": an additional 10 minutes of structured exposure to light over the posterior thorax (lower lung region in back). (10 individuals in each of the two arms). So a total of 20 minutes of intervention each day for those randomized to this group.
-
-
- Data gathering and reporting
- There are 4 "Full Study" days (see Participant's Calendar). These include:
- On Day 1, 11, 21, 31 -
- Responding to an online questionnaire about present symptoms and severity
- Responding to an online mental status exam aimed at assessing cognitive function on each of these more complete study days
- Carrying out an assessment of visual acuity, recording and transmitting results
- Using an innovative (and essentally painless) device to obtain a small blood sample for use in laboratory analysis of markers of inflammation and cellular function
- Measuring vital signs in a protocol designed to assess impact of a very light physical effort, specifically:
- Measuring after 5 minutes at rest - temperature (°C), peripheral oxygen saturation (spO2), Systolic and Diastolic blood pressure (mmHg), Heart Rate (bpm). These masurements are made with supplied equipment, and results recorded and transmitted to the study centre.
- A 6 minute period of specific physical effort.
- A recovery period to assess effects of the brief physical effort, with the same measurements made and recorded at 0, 5, 10 and 15 minutes after the physical effort.
- There are two sequential 10 day periods of intervention with light
- Day 1 to 10
- After 5 measured minutes of rest, the same vital signs listed above are measured.
- Intervention with "Light #1":
-
- For some, this light will supply a placebo or sham wavelength of red light. This is decided by randomization. The actual intervention light follows below.
- For some, 10 minutes of structured exposure to light passed over the head region.
- For others, an additional 10 minutes of structured exposure to light over the posterior thorax (lower lung region in back).
-
- Day 11 to 20
- After 5 measured minutes of rest, the same vital signs listed above are measured.
- Intervention with "Light #2":
- Each enrollee has been presented with 1 lamp holder and two LED light sources. On Day 11, the light initially in the holder ("Light #1") is exchanged for Light #2. Day 11 is referred to as the Crossover Day. If the initial light source had provided a placebo or sham wavelength of light, Light #2 will now be the intervention light with effects under study (and vice versa). Some will be switching (in a blinded and randomized way) to the placebo device. It is important and reassuring to note that ALL enrollees will have access at some point during this study, to the device presumed to be the active intervention.
- For some (selected randomly), 10 minutes of structured exposure to light passed over the head region.
- For others (selected randomly), an additional 10 minutes of structured exposure to light over the posterior thorax (lower lung region in back).
- Day 21 to 30
- These are easy days! They are days off, or days of rest. They are formally included in the protocol to provide a period without any intervention. They are followed by Day 31 which is the final "Full Study" Day.
- No interventions with light
- No vital signs to take. (One is free to do so if desired, but no data need be sent in).
- No other tests or questionnaires.
- Enrollees are free to carry out these days and activities ad lib, as desired. But Day 31 must not be forgotten! It is the final "Full Study" Day used to assess persistance of effects, or lack thereof.
- These are easy days! They are days off, or days of rest. They are formally included in the protocol to provide a period without any intervention. They are followed by Day 31 which is the final "Full Study" Day.
- Day 1 to 10
- On Day 1, 11, 21, 31 -
- There are 4 "Full Study" days (see Participant's Calendar). These include:
Support for Enrollees
All of the above tasks require the equivalent of a "User's Manual" to accomplish the present study with success.
-
- This site is that "User's Manual" - if questions arise that have not been addressed here, every effort will be made to remedy that promptly. Write freely to "Questions@StudyLTCovid.com" for instructions or support.
- To obtain uniformity of measurements made, the required equipment is supplied and explained through Demonstration Videos on this site. These have been structured to include much detail. Some will say "too much detail," but the goal is to be complete and not leave anyone feeling lost with unanswered questions. Scientifically, this should improve results by reducing variability in the measurements carried out by individuals in various locations and not in a laboratory or hospital clinic.
- If no results transmitted: no data: no study. So facilitating this part of the process is essential to protocol success. An individual's results will be sent to specific email addresses. Results are then protected as they arrive, through addition to several databases. All such transmissions respect personal privacy, belong to the present study, and will not be shared in any other way. At the end of the study, results will be presented on this site, and again, without identifying any individual personally.
- Enrolling in the study also involves providing informed consent. This exists to protect enrollees and assure a minimum of risk and a maximum of benefit. It protects the study in that it contributes to trustworthy results. That Informed Consent Process will be presented elsewhere on this site.
- Care has been used to maintain "double-blinding" of study participants including the Principle Investigator. If questions arise, no one will know until after the study has run to completion, whether an intervention or sham light source is being used, for example. Incoming results will not identify until the end of the study, the "arm" and "group" tied to the data. An enrollee must always include her or his "Study ID#" with any data transmmission. Absent that vital identifier, data processing becomes uncertain if not impossible. No further information is provided to avoid countering the study's required blinding and controls.
- While keeping to this "double-blind" state, daily online scheduled meetings (using Zoom) will be offered to enrollees to answer questions and address potential problems if any are discovered. Any research project can be expected to include unanticipated unknowns and surprises. Hopefully, these will be minimal if our pre-study work has been well done.
The components of the present study have been organized into a 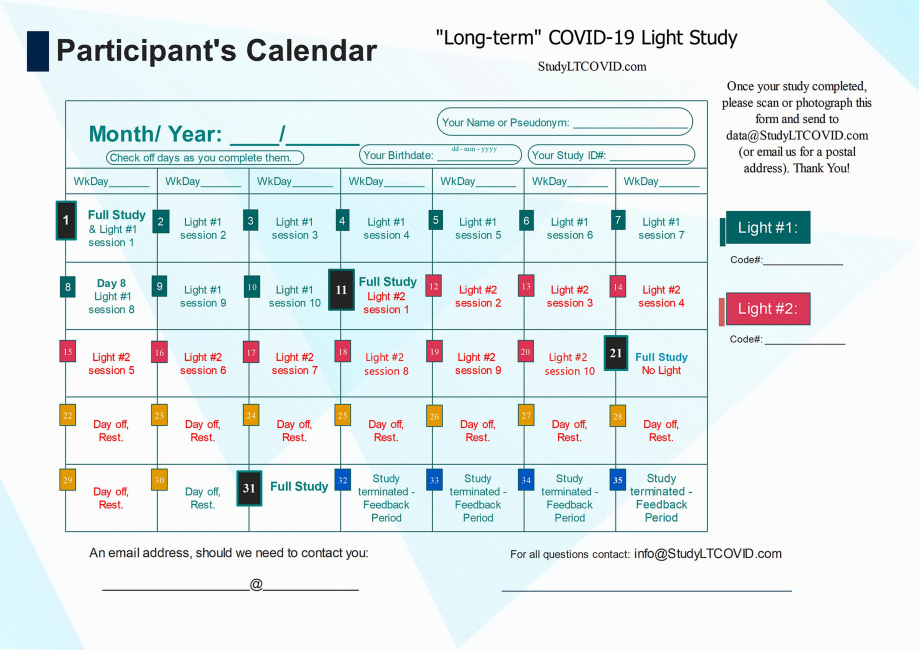
- Enrollees use the above Calendar to check off completed days and see where they are in the study. This for their own use. No need to send it back. (Perhaps a picture or scanned copy would be nice at the end, on Day 31).
- To be reminded of switching lights used on the "Crossover Day," Day 11.
- To get used to their assigned Study ID#____. It is used for transmitting results of vital signs, visual test and other interventions. It is the only identifier used as well to protect enrollee privacy and the randomization process.
Details of What-Happens-When, are provided for the Participant's Calendar.
Most importantly, it serves to crunch what seems like a lengthy Things-To-Do list as presented above, into smaller, daily bite-sized morcels.
Enrollees participate each day in this study, and for which we are grateful.
Enrollees also have a life.
So the study has been structured to minimize interference in an enrollee's day.
<<<< Previous Page (Criteria for Inclusion)
A découvrir aussi
- Do you fit the profile we are looking for?
- Criteria for Inclusion in This Study
- List of 20 most frequent symptoms
